Abstract
Background: Primary mediastinal B-cell lymphoma (PMBCL) is a rare subtype of aggressive B-cell lymphoma. Most relapses occur within the first few months resulting in a dismal prognosis; therefore, it's important to identify primary chemorefractory patients at an early stage, to improve their prognosis. Our group have demonstrated that Circulating Tumor DNA (ctDNA) detected by deep sequencing (DeepSeq) constitute a new non-invasive marker for monitoring response in follicular lymphoma (Jimenez-Ubieto A. et al. ASH 2020). CtDNA monitoring in PMBCL might help to better assess therapeutic response, correct false positive PET/CT results due to residual uptake of the mediastinum and define patients who will benefit from radiation therapy (RT). Here we analyzed the potential value of ctDNA monitoring in 11 PMBCL treated with R-DA-EPOCH between 2018-2020 in the Hospital 12 de Octubre.
Methods: Genomic DNA from paraffin embedded (FFPE) lymph node biopsies were obtained from 11 PMBCL cases at diagnosis. Samples were sequenced with a short length Ampliseq Custom Panel (Thermo-Fisher) designed to cover all coding regions of 56 lymphoma specific genes with an average depth of 700x. After annotation and filtering, 5-8 somatic mutations previously described in lymphoma were selected to be screened in plasma samples. The plasma derived cfDNA was obtained from 8-16mL of peripheral blood collected in EDTA tubes and processed in less than 4h by column purification (QIAamp Circulating Nucleic Acid Kit, Qiagen). A total of 31 different plasma time-points were sequenced in triplicates. On average 78ng (9-224 ng) of cfDNA was used for the DeepSeq of the specific mutations selected in each patient. An average coverage of 236.000x per triplicate was obtained for each mutation. The detection cut-off of 1E-4 was defined based on the LOD obtained in healthy controls donors. 18F-fluorodeoxyglucose (FDG) PET/CT scans were performed on a General Electric Discovery MI Scanner at basal, interim (after 4 cycles), end of induction (EOI) and after radiotherapy (RT).
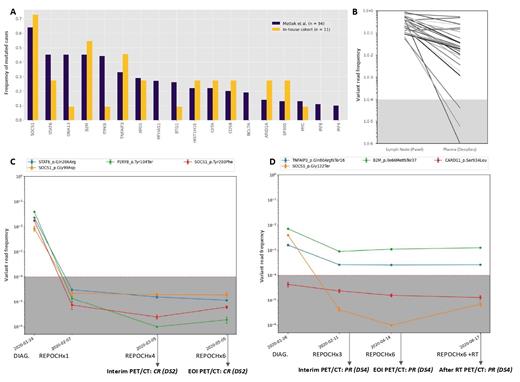
Results: The median age was 33 years and 63.6% were female. Most cases (81.8%) were diagnosed with stage I or II disease and 27.3% cases present with extranodal involvement. On interim PET, 4 patients reached Complete response (CR) and 7 Partial Response (PR, DS4). At EOI, the number of CR turned to 6/11 (55%). All patients in PR at EOI (n=5) and two patients in CR (DS3) with residual mass received RT consolidation (median dose 32Gy). After RT the rate of CR was 91% (10/11). One patient progressed to a classical Hodgkin lymphoma (cHL). None of the patients in CR have relapsed after a median follow-up of 22 months. One patient died due to a mediastinal synovial sarcoma. A total of 125 somatic mutations were detected in the 11 baseline samples with a median of 8 per patient (rank 5-35). The three most frequently mutated genes were SOCS1 (73%), B2M (55%) and TNFAIP3 (46%). Despite the reduced size of our cohort, the mutational frequencies were comparable to the described by Mottok A. et al (Blood 2018, Figure 1A). The DeepSeq of six diagnosis plasma samples showed a lower Variant Read Frequency (VRF) in cfDNA. On those paired samples, 25/28 mutations were detected in plasma, with a median VRF of 2% (0-53%) vs 24% (5.5%-87%) in Lymph nodes (Figure 1B).
The rest of the plasma samples corresponded to 1st cycle (n=5), 4th cycle (n=6), EOI (n=7) and after RT (n=5). After 1 cycle of chemotherapy 3/4 patients who reached CR at EOI had already undetectable ctDNA (Figure 1C). One patient with positive ctDNA after 1 cycle needed RT to convert to CR. All the CR evaluations by PET-TC who had available ctDNA data, presented undetectable ctDNA (n=9). In the EOI analysis all+ patients except the one who progressed to cHL had undetectable ctDNA. In the PR interim evaluations 2/5 had undetectable ctDNA and converted to CR at EOI. Of the three patients with detectable ctDNA, one progressed to cHL (Figure 1D) and 2 needed RT to convert to CR.
Conclusions: Our results demonstrate that disease monitoring using DeepSeq of plasma ctDNA is feasible in PMBCL. Regarding prediction of relapse, the positive predictive value of ctDNA was 100%. An early ctDNA analysis (even after only one R-DA-EPOCH cycle) was able to predict patients in need of RT. Despite the DeepSeq of ctDNA could be useful to disease monitoring to prevent relapse and toxicity reduction by selecting cases in need of RT, more patients are necessary to draw meaningful conclusions.
Martín-Muñoz: Altum sequencing: Current Employment. Dorado: Altum sequencing: Current Employment. Heredia: Altum sequencing: Current Employment, Current equity holder in publicly-traded company. Rufian: Altum sequencing: Current Employment. Canales: Incyte: Consultancy; iQone: Honoraria; Takeda: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Celgene/Bristol-Myers Squibb: Consultancy, Honoraria; Sanofi: Consultancy; Eusa Pharma: Consultancy, Honoraria; Sandoz: Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Speakers Bureau; Gilead/Kite: Consultancy, Honoraria. Juarez: Altum sequencing: Current Employment. Sanchez: Altum sequencing: Current Employment. López-Muñoz: Amgen: Consultancy. Ayala: Incyte Corporation: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Celgene: Honoraria. Martínez-López: Janssen, BMS, Novartis, Incyte, Roche, GSK, Pfizer: Consultancy; Roche, Novartis, Incyte, Astellas, BMS: Research Funding. Barrio: Altum sequencing: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal